

InteReg Scientific Goals
Traumatic injuries of the central nervous system (CNS), such as brain or spinal cord injuries, do not regenerate efficient and therefore often lead to severe and permanent disability. The poor regenerative capacity of the CNS is due to multiple obstacles after injury: 1. a glial scar forms at the lesion site and acts as a barrier for axonal regrowth, 2. myelin and axonal debris send inhibitory cues to axons, which also prevents regrowth, 3. there is no guidance mechanisms allowing axons to regrow in the right direction to reconnect with their former target, 4. Remyelination is not very efficient. These multiple obstacles preclude functional recovery after a CNS injury.
Multiple Sclerosis (MS) is a demyelinating autoimmune disease influenced by genetic, viral and environmental factors in which oligodendrocytes (OLs), the myelinating glia of the CNS, are attacked by immune cells, resulting in demyelinating lesions where axons lose their myelin sheath. Early in the disease, OL precursor cells (OPCs) are often able to migrate to the lesion site and remyelinate demyelinated axons. However, as patients age and the disease progresses, this process becomes less efficient. The loss of myelin leads to an impairment of CNS functions, which can become permanent if axons degenerate due to long-term demyelination. While numerous therapies have been developed to slow down the immune attack frequency, very little progress has been made in regeneration therapies. As a result, there is currently no drug available to MS patients to promote regeneration after lesion.
Our Team aims at developing models and tools for a new generation of therapies based on precision-engineered artificial biomaterials to promote CNS regeneration after a traumatic injury or in the context of MS.
In Aim 1, we will develop ECM mimics that have bioinstructive cues, bio- and mechano-responsive properties, and which have the capacity to provide and deliver pro-regenerative factors that will be used by living cells on demand. Project Leads: Andreas Walther, Pol Besenius, Claire Jacob, Martin Heine

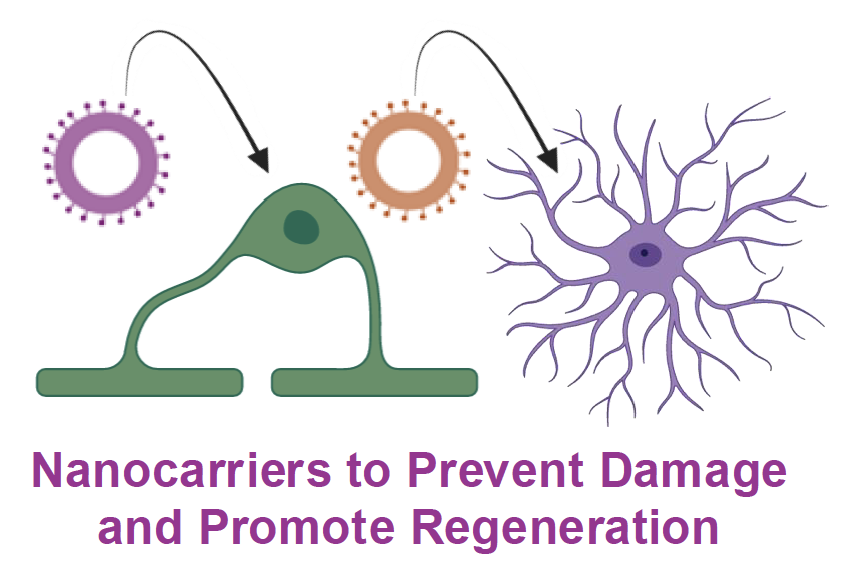
in Aim 2, we will generate nanocapsules that are targeted to specific cells to reprogram them into repair-promoting phenotypes. Project Leads: Katharina Landfester, Volker Mailänder, Claire Jacob, Maja Papic, Benedikt Berninger, Tanja Weil
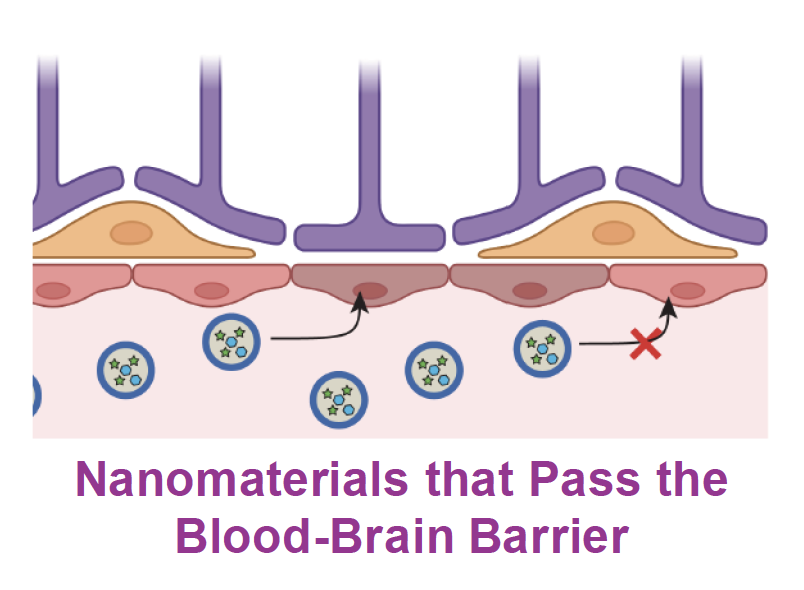
Because target cells are located in the CNS, in Aim 3, we will work at engineering nanomaterials that cross the BBB. Project Leads: Katharina Landfester, Volker Mailänder, Andreas Walther, Ari Waisman
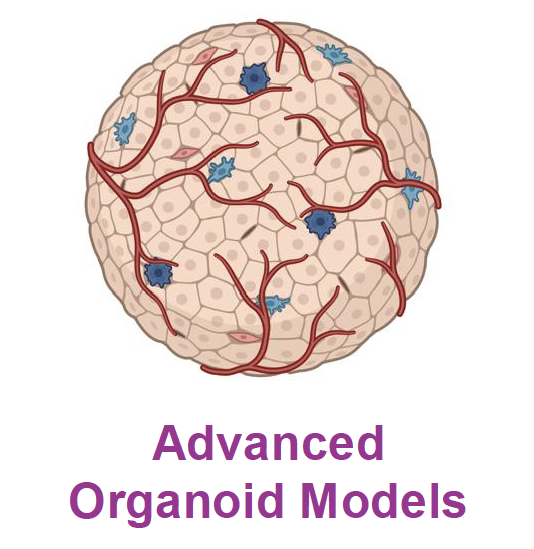
In Aim 4, we will use established and generate new animal test surrogate models to test our biomaterials. We have already developed microfluidic cell-based models and human cell-derived brain organoids, and in InteReg we will use 3D printing to generate more complex models of vascularized brain organoids to test the passage of our nanomaterials through the BBB, their delivery to specific cells, and their therapeutic potential. Project Leads: Maria Villiou, Andreas Walther, Himanshu Mishra, Beat Lutz, Benedikt Berninger, Martin Heine, Claire Jacob
Illustrations made with BioRender